Data Safety and Monitoring Boards Should Be Required for Both Early- and Late-Phase Clinical Trials - ScienceDirect

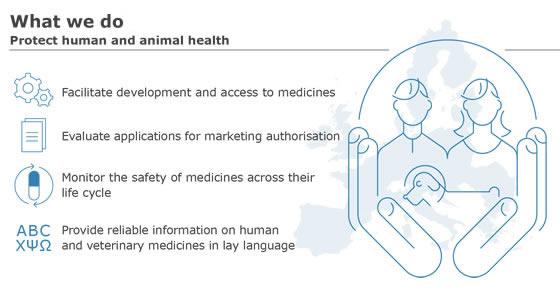
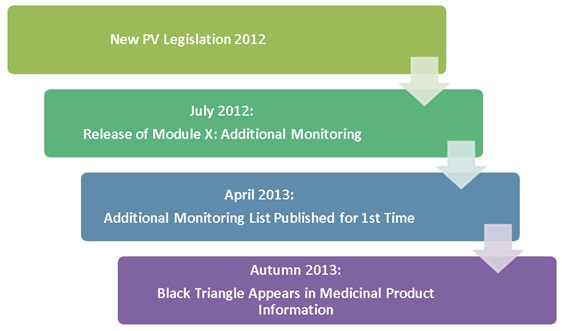
News from the EACPT: New European Medicines Agency advice on black triangle prompts for medicines monitoring

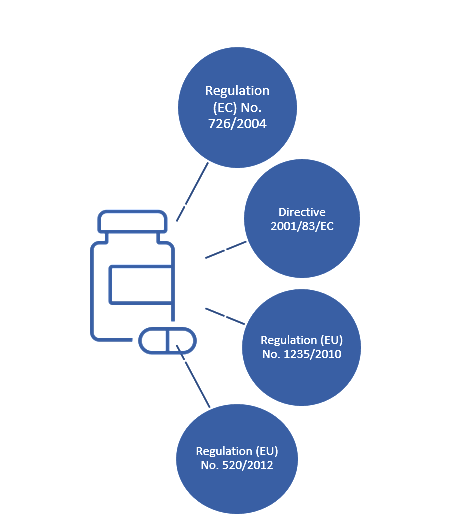
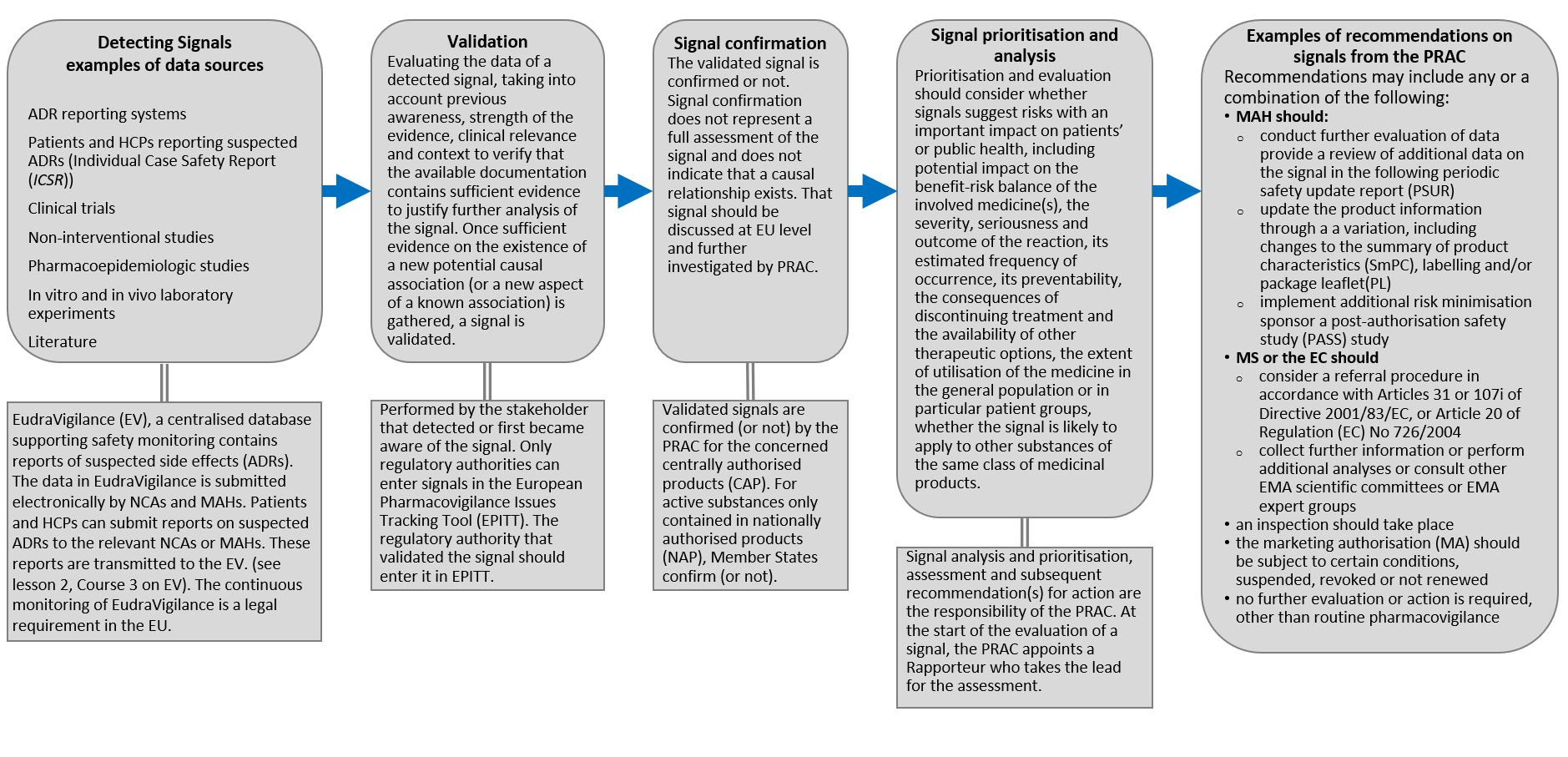
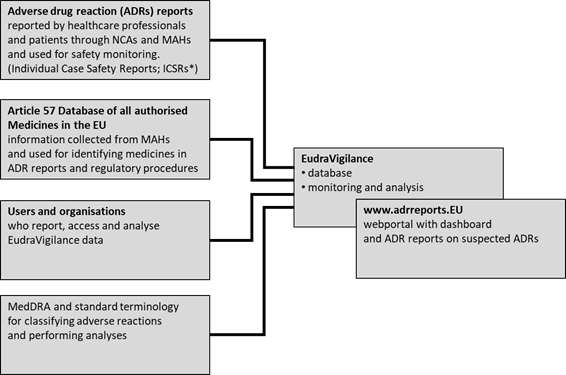
TAIEX Workshop on the Implementation of EU Pharmacovigilance Legislation - BELGRADE CLAUDIA PANAIT TAIEX Expert – European Commission Legal Adviser. - ppt download